Elemental Shift: Periodic Table Gets Weight Changes
Ten elements that help make up the universe, including the carbon our biology is based on and the oxygen in the air we breathe, are now getting changed in an unprecedented way — they are getting their very atomic weights altered.
Scientists have not invented some magical way to transform the masses of all these elements. Instead, they are updating what are often thought of as constants of nature on the periodic table.
"For more than a century-and–a-half, many were taught to use standard atomic weights — a single value — found on the inside cover of chemistry textbooks and on the periodic table of the elements," said physicist Michael Wieser at the University of Calgary. "As technology improved, we have discovered that the numbers on our chart are not as static as we have previously believed."
The standard atomic weight of an element, which is made up of one type of atom, is based on the mass of its atoms. The problem that scientists are now addressing is rooted in the fact that these atoms don't always have the same masses. While all the atoms making up an element have the same number of protons, elements have variants known as isotopes that possess different numbers of neutrons in their nuclei, making some lighter or heavier than others.
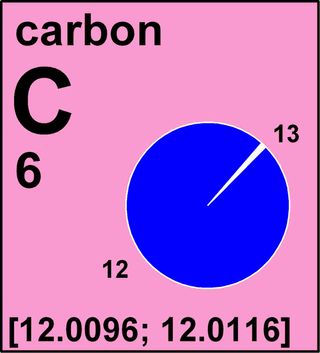
Certain elements have more than one stable isotope. For instance, carbon has two — carbon-12 and carbon-13. (The numbers in each isotope reveal how many particles they have in their nuclei — carbon-12 has six protons and six neutrons.) In the past, to give a standard atomic weight for these elements, scientists averaged out the atomic weights of these isotopes based on how common those isotopes are — the more plentiful an isotope was, the bigger a role it played in the standard atomic weight.
However, the abundance of an isotope can vary in nature, leading to variations in an element's atomic weight. For example, sulfur is commonly known to have a standard atomic weight of 32.065, but its real atomic weight can be anywhere between 32.059 and 32.076, depending on where the element is found.
These small variations in an element's atomic weight can weigh heavily on research and industry. For example, precise measurements of the abundances of carbon isotopes are used to determine purity and source of food, such as honey and vanilla. Isotopic measurements of nitrogen, chlorine and other elements help trace pollutants in streams and groundwater. In sports doping investigations, scientists can identify performance-enhancing testosterone in the human body because the atomic weight of carbon in natural human testosterone is higher than that in pharmaceutical testosterone.
"There's a lot of practical information we can get from knowing atomic weight, all these significant problems and issues where knowing atomic isotope abundance can play a key role," Wieser told LiveScience. He serves as secretary of the International Union of Pure and Applied Chemistry's (IUPAC) Commission on Isotopic Abundances and Atomic Weights, which oversees the evaluation and dissemination of atomic-weight values.
Now, for the first time in history, the standard atomic weights of 10 elements — hydrogen, lithium, boron, carbon, nitrogen, oxygen, silicon, sulfur, chlorine and thallium — will get expressed in a new way that will more accurately reflect how these elements are found in nature. Instead of single values, they will get expressed as intervals, having upper and lower bounds, to more accurately convey variations in atomic weight. For instance, carbon's standard atomic weight is listed as an interval between 12.0096 and 12.0116.
The other elements on the periodic table remain the same, as elements with just one stable isotope do not show variations in their atomic weights. For example, the standard atomic weights for fluorine, aluminum, sodium and gold are constant, and their values are known to more than six decimal places.
These changes might seem confusing to students and scientists. Which number should they use on a test, or in the lab? Ultimately, it will depend on the element and the context.
If they just want to perform a simple calculation involving these 10 elements, they can use a single value called a conventional atomic weight, Wieser said. If they need more precision — more decimal places in the number — they can look up an atomic-weight value for the specific context they have in mind. For instance, "boron in seawater has a very narrow atomic-weight range, so I could select a value of 10.818," research chemist Tyler Coplen, director of the U.S. Geological Survey's Reston Stable Isotope Laboratory, who was worked on these changes for the past 15 years, told LiveScience.
Coplen and Wieser said they were completely surprised about the attention this change has received.
"People might remember sitting in a chemistry class with the periodic table hanging on the wall, and after seeing that some elements such as sodium or gold were measured to an incredible precision, wondered why others such as sulfur and lead weren't measured to the same precision," Wieser said. "Now this change might answer that."
These changes became official when IUPAC published them online Dec. 12 in the journal Pure and Applied Chemistry.
- Twisted Physics: 7 Recent Mind-Blowing Findings
- Top 10 Greatest Mysteries in Science
- 10 Events That Changed History
Live Science newsletter
Stay up to date on the latest science news by signing up for our Essentials newsletter.

Most Popular

By Harry Baker

By Ben Turner

By Jamie Carter